...
The air around us as dominos: Viscous Flow
Sound is motion
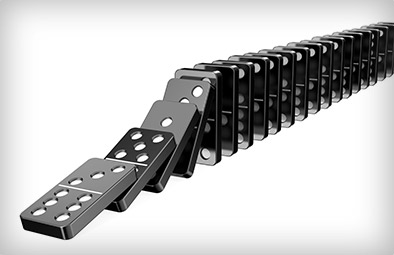
Remember: molecules are tiny, and there's a lot of them in the air around you—there are more molecules within a literal hair's width of a pinhead than one could possibly count. And what are they doing? Bouncing into each other. Knock enough molecules in one direction and those molecules will knock into the next like dominoes, and that's pretty much how sound happens:
Sound is waves of rapid pressure change, and no molecules need to travel from the speaker to the ear to hear. Watch the red dots in the above animation, see how they just oscillate back and forth? Now compare that to the smoke going back and forth with the sound waves, same thing:
The speakers moved enough air to extinguish the flames, but it wasn't the air from the speaker that put out the candles. The air from the speaker started the domino chain, but it was the air already next to the flame that put the flame out.
And the domino chain that is the sound wave moves through the air so fast that it looks like all the flames went out at once, but the flames get extinguished one after the next, at the speed of sound, but the speed of sound is simply too fast to see. So the sound has direction, but the air doesn't flow. This lack of airflow is unlike blowing out a flame, where the air itself moves in a particular direction.
Blowing is flowing
Blow out a flame and the continuous stream from behind makes the air flow from one place to another—it's like pushing a crowd from the back. Keep pushing and it keeps flowing, as long as it has somewhere to go. we call this behavior viscous flow.
Suction pumps create flow in just the opposite way: remove some molecules and the ones next to it fall into the empty space left behind and, just like the dominoes, that next door molecule leaves behind it's own void that gets filled by other molecules, and so on and so forth.
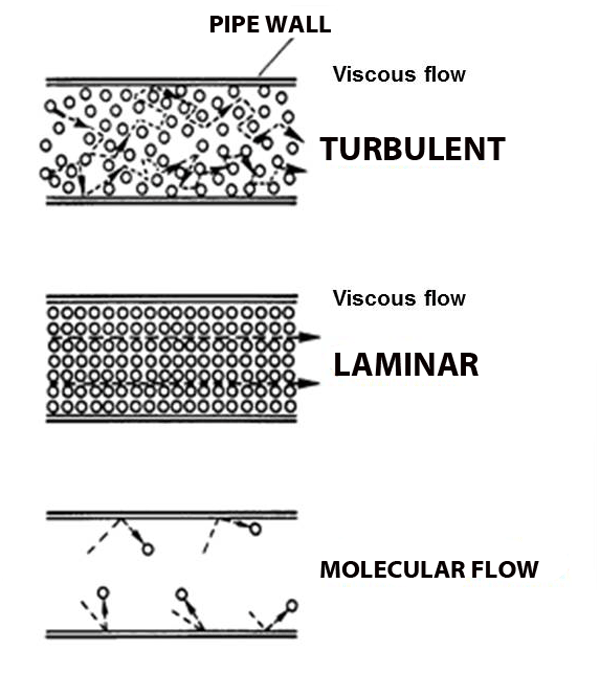
Both blowing and sucking create the domino-chain-like behavior known as viscous flow, and it's what we're used and expect because we live out our entire lives in this gas we call 'air', always at atmospheric pressure. When viscous flow is slow it can be smooth (laminar) like the smoke from the flame, and when the flow gets fast it gets rough (turbulent), like the blown smoke. But what happens when the pressure gets so low that molecules hardly run into each other? No more domino chains, no more viscous flow. Molecular Flow.
Molecular flow: air hockey analogy
...