Intro
This is a beginner's beginner guide to vacuum theory. Into to vacuum Theory aims to reach a broad audience by using brief, conversational descriptions and avoiding the use of math or units; no science background needed. It incrementally introduces some of the concepts and language used in vacuum technology. Hopefully this lays a solid groundwork for future learning.
This training document starts off zoomed out and oversimplified, and builds off itself as it goes, using vocab defined earlier to help define newer terms. It ends, still zoomed out and oversimplified, but less so. So take note: these topics run much, much deeper than described within this document; further discussions can be found within the other pages of LCLS Vacuum Support Training, within textbooks, and most of all within your coworkers (go talk with them!).
Side note: Most of the pictures here are linked to external material, including: videos, articles, slideshows, and product pages. Click to explore; though they aren't necessary, and can be tangential or beyond the scope of this introductory guide.
Happy reading!
Starting at the bottom, Setting the scene
Atoms
Atoms, totally a thing! they're small. really small. a million of them fit across a single human hair. tiny, but they still take up space.
they often like to group up with other atoms to form molecules.
Gasses
Hey so gasses exist. You're breathing some right now.
What are gasses made of? molecules. Usually as a mix of different molecule types. Air is a mix of over ten types of molecules (mostly nitrogen and oxygen).
People work very hard to collect gasses that aren't mixed.
What are those gas molecules doing? mostly just bouncing around. –off each other, off the walls
How fast are they moving? fast. really fast. usually over a thousand miles per hour (you are being pelted by molecules right now).
The speed of individual molecules depend on their temperature and mass: cold things fly slower, heavy things fly slower.
but that speed is only for individual molecules; the gas as a whole has no great speed or direction because the molecules bounce off each other randomly in every possible direction.
How many molecules are in any given space? a lot! the room you're sitting in has about a gazillion gas molecules in it.
BUT: the number of molecules in a room depends on the pressure of the gas in the room: fewer molecules = lower pressure.
Gas Pressure
When a gas molecule bounces off of something it pushes on that something. If that something is another gas molecule that other molecule goes flying off. If that something is much bigger, for example a metal box, the molecule will push on the box just the same, but the box will hardly budge, it's way too big.
Ah but what happens when we add more molecules to the box? All of those little bounces add up. And that's pressure: the push from all the molecules. Add enough molecules and the pressure will rise so high that the box might pop!
Now what happens if we remove, instead of add, molecules? Vacuum.
Vacuum
When the pressure in the box is lower than the air around us, we call it a relative vacuum.
When there are absolutely no molecules whatsoever in a space, we call it a perfect vacuum.
but a perfect vacuum never happens. All vacuum chambers leak. And even in the furthest reaches of space, a stray molecule here and there flies past; though it probably won't hit another molecule for many lifetimes.
Now, getting into the nitty gritty:
Behaviors of gasses
Mean Free Path
The average distance that a gas molecule can travel before colliding with another gas molecule is called the mean free path.
as pressure drops, mean free path increases, because there are less molecules to run into. This effects the behavior of gasses, discussed below:
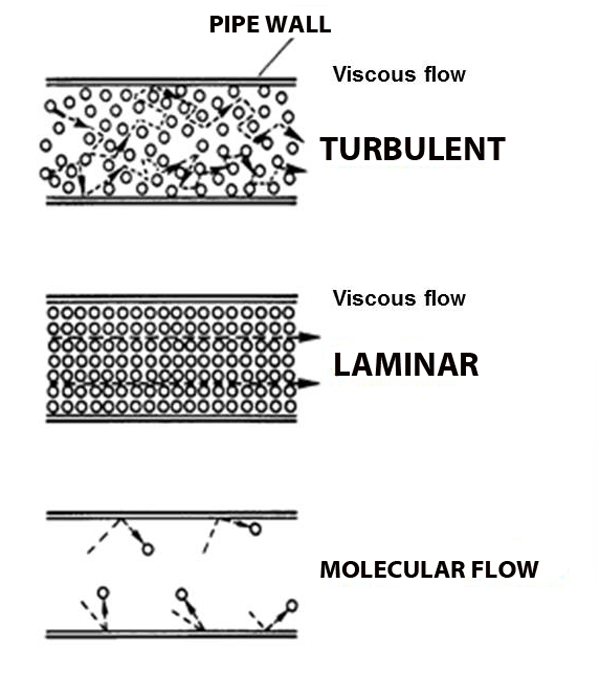
Viscous flow
Under normal atmospheric conditions– gas molecules are constantly running into each other (short mean free path) and bouncing off.
In fact, when you blow out a candle it's not the molecules from your lungs that put out the flame, there are too many air molecules in the way; your lung-air molecules merely knock forward the air molecules in front of your mouth, which knock forward the air molecules above the cake, which in turn knock the air molecules in the flame away from the wick, taking their heat with them, and extinguishing the flame. And pumps create suction in just the opposite way: remove some molecules and the ones next to it fall into the void, leaving their own void behind them, which gets filled by other molecules, and so on and so forth. This domino-chain like behavior is called viscous flow, and it's what we're used to.
But what happens when the pressure gets so low that molecules hardly run into each other? Molecular flow.
Molecular Flow
Imagine the world's largest air hockey table, you place a few pucks on the table, and hit them in random directions. They'll hit each other sometimes, but they're much more likely to hit the wall. And it's just as likely to bounce back into your own goal as it is to bounce into the opponent's goal. Now replace the flat of the table with the space inside a vacuum chamber, and replace the pucks with molecules; this is molecular flow: when a molecule is more likely to hit a chamber wall than it is to hit another molecule, and unlike viscous flow, there is no general directionality to the flow (see figure above).
Pumping in a molecular flow
For a pump to create suction, it needs enough molecules around for the domino-chain behavior of a viscous flow, so in molecular flow a pump cannot pull air. But if that pump Can't suck, what does it do? It traps. Like the goal on an air hockey table traps the puck when it flies in. But there's nobody knocking molecules towards the pump like there would be in air hockey, It's random.
How likely is a molecule to randomly fly into a pump that traps? That depends on how big the tube is leading to the pump.
Tube Size and Molecular Conductance
A long/narrow tube restricts gas flow more than a wide/short tube. The rate of gas flow through a tube is called the tube's conductance; the long/narrow tube has the lower conductance of the two.
Sadly adding a bigger pump to suck faster though the tiny tube only works in viscous flow. In molecular flow, the pump will only ever remove molecules at the rate they naturally fly through the smallest/longest tube in leading to the pump. The lowest conductance point sets the pace. =(
Mixing Gasses: Partial pressures
So gasses mix. How does mixing gasses change pressure? The pressures add up. Here's an example:
In the mixed chamber the oxygen and nitrogen are still contributing the same pressures they had when they were separate. Those contributing pressures are called partial pressures. Oxygen's partial pressure in the mixed chamber is 2 units of pressure.
Stuck on walls: Condensation and Evaporation
Ok, so you know how I said molecules bounce off walls? Not actually true. I lied to keep it simple, but I think you're ready for the truth: It turns out that any time a gas molecule hits a surface it actually sticks to that surface, just like how your breath fogs the mirror; this called adsorption (not to be confused with absorption).
And also just like fog on a mirror vanishes over time, those stuck molecules will eventually jump back off of the surface (I.e. evaporate, outgas, desorb), flying of in a random direction just as fast as it was when it hit the surface.
How long does the molecule stay stuck before desorbing from the surface?
Well that depends on the molecule, the temperature, and the what the surface is made of; and it can happen anytime between almost instantly and basically never.
If a type molecule tends to jump off quickly, from a given surface at a given temperature, it will find it's way into a pump pretty quickly, and the pressure will drop. And if a type molecule tends to jump off rarely, from a given surface at a given temperature, the pressure also goes down, it's not even contributing the the pressure, because it's not a gas, it's trapped on the surface!
The trouble for lowing pressure comes when a molecule jumps around enough to raise the pressure, but not enough to easily find it's way into the pump.
Contamination: molecules sticking to walls intermittently
main culprits:
water - from moisture in the air
hydrocarbons (oils, plastics)
you know what's really oily? You.
how to defeat contamination
prevent it!
use gloves, change them frequently
clean anything going inside the vacuum
opening the chamber? pump nitrogen into it - prevents water from getting in
nitrogen gas has no water vapor, unlike air.
bake it
making the chamber hot will get help those sticky molecules (water and oil) move along faster
detailed discussion the see pages on baking
Leaks
all vacuum chambers leak
really big leaks can be heard, usually from something like a mechanical connection that wasn't tightened
and then something, like a hair, sitting on a gasket
gas permeability
for detailed discussion see the Leak Checking pages
Units of pressure, log scales
UHV
Vacuum Chambers
it's just a box for holding nothing.
And this topic is really beyond what this document is about (introduction to theory), but keep exploring! For more details on Vacuum System parts and how they actually work, see the confluence pages on vacuum components
In Depth slides: Vacuum Science and Technology for Accelerator Vacuum Systems










/GettyImages-1061851344-670639e17b314169bf6f62ba09bfd259.jpg)