let's start waaaay zoomed out and grossly oversimplified:
atoms:
atoms, totally a thing! they're small. really small. a million of them will fit across a single human hair.
they often like to group up with other atoms to form molecules. cool.
gasses:
Hey so gasses exist. –you're breathing some right now.
what are gasses made of? molecules.
what are those molecules doing? mostly just bouncing around. –off each other, off the walls
how fast are they moving? fast. really fast. usually over a thousand miles per hour (you are being pelted by molecules right now).
the speed of an individual molecule depends on its temperature and its mass: cold things fly slower, heavy things fly slower.
but that speed is just for individual molecules; the gas as a whole has no great speed or direction because the molecules bounce off each other randomly in every possible direction.
how many molecules are in any given space? a lot! the room you're sitting in has about a gazillion gas molecules in it.
BUT: the number of molecules in a room depends on the pressure of the room: lower pressure = fewer molecules.
That's gasses.
ok starting getting into the nitty gritty:
mean free path: the average distance that a gas molecule can travel before colliding with another gas molecule.
as pressure drops, mean free path increases, because there are less molecules to run into.
behaviors of gasses:
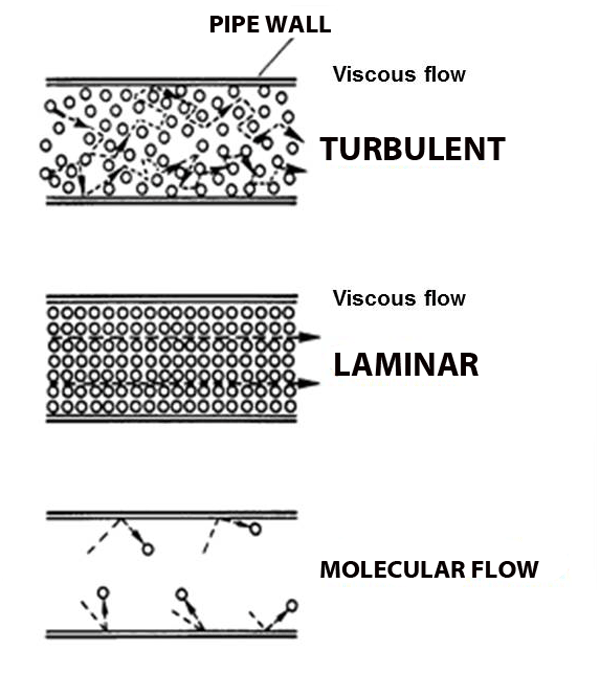
viscous flow VS molecular flow
viscous flow is how gasses work regularly, like water from a pipe. But when the mean free path grows long enough, due to dropping pressure, there aren't enough molecules around for the gas to act like water from a pipe, the gas is now in molecular flow.
Now let's relate this to our vacuum chambers: They start at atmospheric pressure, which is in viscous flow. We turn on a pump, and after a few minutes, the pressure has dropped enough to be in molecular flow. And under molecular flow, wind doesn't blow and pumps don't suck, the atoms are just too far apart!
So how does a pump pump if it can't suck? It traps. It's just a trap waiting for a molecule to fly in, and once it does, it can't get back out. Pumping achieved!
pumping
conductance
contamination
molecules sticking to walls intermittently
main culprits:
water - from moisture in the air
hydrocarbons (oils, plastics)
how to defeat contamination
prevent it!
use gloves, change them frequently
clean anything going inside the vacuum
opening the chamber? pump nitrogen into it - prevents water from getting in
nitrogen gas has no water vapor, unlike air.
bake it
...
In Depth slides: Vacuum Science and Technology for Accelerator Vacuum Systems