...
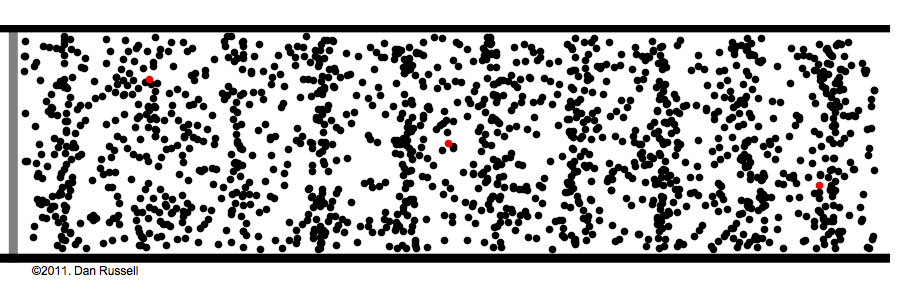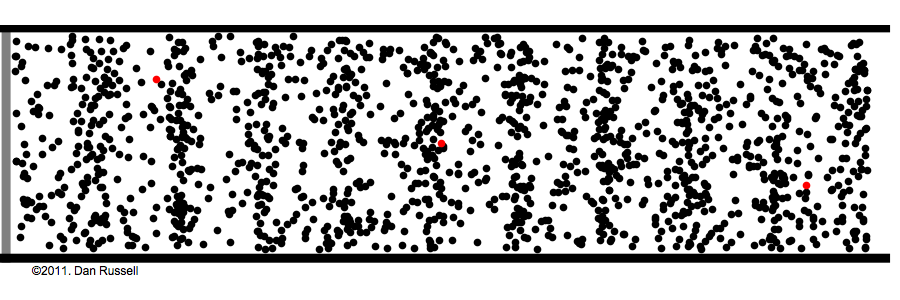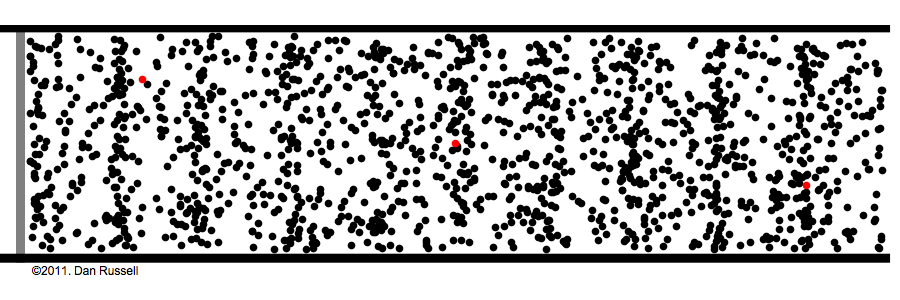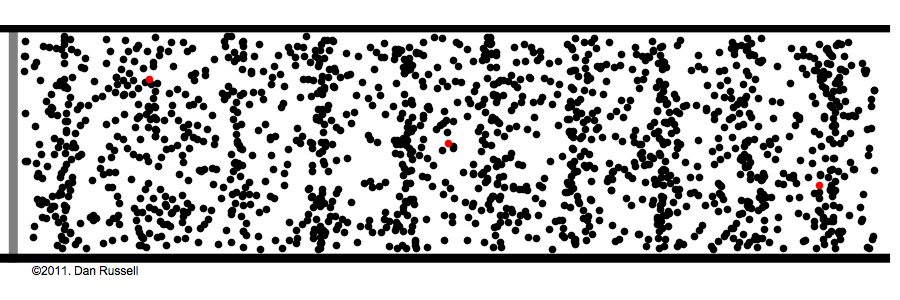
What are those gas molecules doing? mostly just bouncing around. –off each other, off the walls. T
...
How fast are they moving? fast. really fast. usually over a thousand miles per hour (you are being pelted by molecules right now).
...
but that speed is only for individual molecules; the gas as a whole has no great speed or direction because the molecules bounce off each other randomly in every possible direction.
How many molecules are in any given space? a lot! the room you're sitting in has about a gazillion gas molecules in it (the box in the above animation represents an area much tinier than a grain of sand).
...
Remember: molecules are tiny, and there's a lot of them in the air around you—there are more molecules within a literal hair's width of a pinhead your nose than one you could possibly count in a lifetime. And what are they doing? Bouncing into each other. Knock enough molecules in one direction and those molecules will knock into the next like dominoes, and that's pretty much how sound happens:
Sound is waves of rapid pressure change, and no molecules need to travel from the speaker to the ear to hear. Watch the red dots in the above animation, see how they just oscillate back and forth? Now compare that to the smoke going back and forth with the sound waves, same thing:
...
And the domino chain that is the sound wave moves through the air so fast that it looks like all the flames went out at once, but the flames get extinguished one after the next, at the speed of sound, but and the speed of sound is simply too fast to see. So the sound has direction, but the air doesn't flow. This lack of airflow is unlike blowing out a flame, where the air itself moves in a particular direction.
...
Suction pumps create flow in just the opposite way: remove some molecules and the ones next to it fall into the empty space left behind and, just like the dominoes, that next-door molecule leaves behind it's own void that gets filled by other molecules, and so on and so forth.
Both blowing and sucking create the domino-chain-like behavior known as viscous flow, and it's what we're used to and expect because we live out our entire lives in this gas we call 'air', always at atmospheric pressure. When viscous flow is slow it can be smooth (laminar) like the smoke from the flame, and when the flow gets fast it gets rough (turbulent), like the blown smoke. But what happens when the pressure gets so low that molecules hardly run into each other? No more domino chains, no more viscous flow: Molecular Flow.
...
Imagine the world's largest air hockey table, you place a few pucks on the table, and hit them in random directions. They'll hit each other sometimes, but they're much more likely to hit the wallwalls. And it's just as likely to bounce back into your own goal as it is to bounce into the opponent's goal. Now replace the flat of the table with the space inside a vacuum chamber, and replace the pucks with molecules; this is molecular flow: when a molecule is more likely to hit a chamber wall than it is to hit another molecule, and there is no general directionality direction to the flow (see figure the flow. check out the molecular flow figure (above the air hockey table), some arrows point forwards and some backwards), unlike viscous flow where all the arrows point generally forwards.
Pumping in a molecular flow
For a pump to create suction, it needs enough molecules around for the domino-chain behavior of a viscous flow, so in molecular flow a pump cannot pull air. But if that pump Can't suck, what does it it do? It traps. Like the goal on an air hockey table traps the puck when it flies in. But there's nobody knocking molecules towards the pump like there would be in air hockey, It's alllll random.!
Well if a molecule getting trapped behind a pump is random, How likely is a molecule to randomly fly into a pump that traps? That depends on how big the tube is leading to the pump.
Tube Size and Conductance
...
Sadly adding a bigger pump to suck faster though the tiny tube only works in viscous flow. In molecular flow, the pump will only ever remove molecules at the rate they naturally fly through the smallest/longest tube in leading to the pump. Putting a bigger pump on the same tube won't pump any faster. The lowest conductance point sets the pace. =(
...
Ok, so you know how I said molecules bounce off walls? Not actually true! 😬 I lied to keep it simple, but I think you're ready for the truth: It turns out that any time a the only way wall-molecule interaction plays out. most of the time a gas time any gas molecule hits a surface it actually sticks to that surface, just if only temporarily, like how your breath fogs the a mirror; this called adsorption (not to be confused with absorption).
And also just like fog on a mirror vanishes over time, those stuck molecules will eventually jump back off of the surface (Ii.e. evaporate, outgas, desorb), flying of off in a random direction just as fast as it was when it hit the surface..
How long a molecule stays stuck before desorbing from a surface that depends on the type of molecule, the temperature, and the what the surface is made of. Evaporation of a molecule can happen anytime any time between almost instantly and basically never. And we'll never be a able to predict the moment exactly, but we usually have a general idea for how long, based on things like temperature, pressure, and material.
When a molecule is flying around a vacuum chamber, it's contributing to the total pressure, but a molecule is trapped on the surface doesn't contribute to pressure because it's not a gas, it's trapped on the that surface! So if a type molecule tends to jump off surfaces rarely, the pressure can stay low. And also if a certain type molecule tends to jump off quickly (from a given surface at a given temperature) it will hop around enough to quickly find its way into a pump, again the pressure drops.
The trouble for lowering pressure comes when a given type molecule jumps around enough to raise the pressure, but not enough to easily find it's way into the pump.
Contamination: molecules sticking to walls intermittently
molecules jumping around intermittently keeping the pressure too high
Main culprits
Water - from moisture in the air
attaches to chamber surfaces
Hydrocarbons (oils, plastics)
what if: leave a piece of plastic inside the vacuum chamber (oops!), then pump it down:
the plastic will outgas and coat the chamber walls with a fine layer of hydrocarbons
you know what's really oily? You.
Dust
it's all around us, slowly settling on surfaces.
Dust always matters. We try to avoid it. Sometimes a lot. Sometimes not so much.
how to defeat contamination
Prevent it!
- use gloves, change them frequently
- clean anything going inside the vacuum chamber
- opening the chamber? pump nitrogen into it first, keep the nitrogen flowing while the chamber is open - prevents water from getting in
nitrogen gas has no water vapor, unlike air.
- worried about dust? don't let it get in your chamber!
wear special dust-free robes and hats. Do vacuum work in a special dust free room.
Bake it
making the chamber hot will get help those sticky molecules (water and oil) to jump around more
we wrap the chamber in electrical heating tape to heat it from the outside in. it takes days.
detailed discussion see pages on baking
water: 150°C
hydrocarbons: 250°C
Leaks: always happening, hopefully tiny
sources of leaks
leaking in from the outside
- really big leaks can be heard, usually from something like a seal that wasn't tightened
- smaller leaks come from various places, often for example a hair sitting on a seal or ????
- tiny tiny leaks: individual gas molecules wriggle their way through materials (gas permeability), especially rubber, making a very slow leak.
all vacuum chambers leak, but the leak doesn't have to come from outside the chamber...
leaks from within the chamber
gas trapped in a pocket slowly leaks into the rest of the chamber - a virtual leak, eventually it runs out, but can take weeks.
The above diagram's screw has a hole drilled through the middle to allow the trapped gas to escape quickly. these are called 'vented screws' and are used commonly inside vacuum chambers. But there are other places gas can get trapped besides at the bottom of screw holes.
How do we find small leaks?
with helium.
For detailed discussion on leaks, how to find them, and what to do about them see the Leak Checking pages
Time to talk numbers (sorry)
Units Used with Pressure
we use torr to describe the pressure of our vacuum systems
image of vacuum guage used here at SLAC
we use psi (pounds per square inch) to describe the pressure of our compressed systems
compressed: air, nitrogen, helium, argon, etc...
we use liters per second (l/s) to describe the rate of gas flowing through something
Log Scales (making numbers lie)
Not this log scale:
The log scale I'm talking about... It's math. sorry.
Log scale allows people to create graphs that show tiny things next to giant things really well by stretching out the distance between tiny numbers, and compressing the distance between huge numbers. The powers of 10 does this really well.
Exponents and powers of 10
The little number above the 10's, called the exponent, is the number of zeros.
...
A negative exponent is how many zeros past the decimal place.
What's the difference between 107 and 103? the same difference between 10,000,000 and 1,000: over 9 million.
What's the difference between 10-7 and 10-3? the same difference between 0.00000001 and 0.0001: less (much less) than 1
-Different, but in both cases the difference is 4 orders of magnitude. look at the exponents: 7 - 3 = 4, tiny or huge, doesn't matter.
Linear vs Logarithmic
In linear counting the numbers increase one at a time. It's what we're used to. t's how we normally count: 1, 2, 3, 4, 5 etc...
In logarithmic (i.e.: log) counting the numbers increase by bigger amounts each time. Log counting is strange, counterintuitive and, unfortunately, useful.
the log scale and the linear scale both start around 1 go up to 100,000. But what's different is how they get from 1 to 100,000. The second number in linear scale is 20,000, but in log scale the second number is only 10. Also in log scale the second to last number is 10,000, still much less than the second number in the linear scale: 20,000.
Common units that use log scales: sound (decibels: dB), earthquakes (Richter magnitude)
UHV: how teeny tiny
Ultra-High Vacuum (UHV) is an official term for really really fancy vacuum of a certain (extremely low) range of pressures. And it's the type of vacuum we care about the most.
For molecules in UHV the mean free path is at least 200 miles. Any molecule in UHV is going to see the chamber walls thousands of times before it will see another molecule.
Vacuum Chambers
it's just a box for holding nothing.
Sadly the details of vacuum chambers and their construction is really beyond what this document is about (introduction to theory), but keep exploring!
...
Check out the Application of Vacuum Theory page to see how we deal with troublesome molecules.
Here is a presentation created by Dan Peterswright that introduces some vacuum concepts and walks through Gas Laws. It also offers a few examples that illustrate the effects of pressure differentials created by establishing vacuum in flexible couplings and brief examples that illustrate the related gas laws.
| View file | ||||
|---|---|---|---|---|
|