...
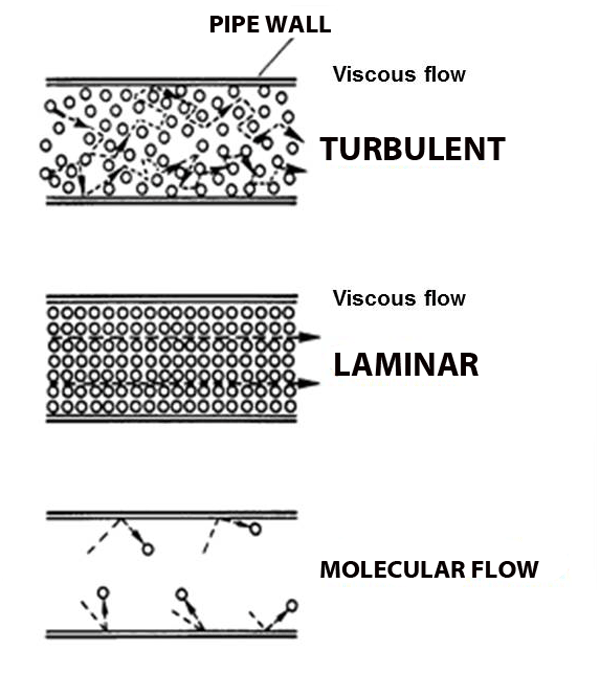
Both blowing and sucking create the domino-chain-like behavior known as viscous flow, and it's what we're used and expect because we live out our entire lives in this gas we call 'air', always at atmospheric pressure. When viscous flow is slow it can be smooth (laminar) like the smoke from the flame, and when the flow gets fast it gets rough (turbulent), like the blown smoke. But what happens when the pressure gets so low that molecules hardly run into each other? No more domino chains, no more viscous flow. Molecular Flow.
...
Low pressure flow: air hockey analogy
Imagine the world's largest air hockey table, you place a few pucks on the table, and hit them in random directions. They'll hit each other sometimes, but they're much more likely to hit the wall. And it's just as likely to bounce back into your own goal as it is to bounce into the opponent's goal. Now replace the flat of the table with the space inside a vacuum chamber, and replace the pucks with molecules; this is molecular flow: when a molecule is more likely to hit a chamber wall than it is to hit another molecule, and there is no general directionality to the flow (see figure above the air hockey table), unlike viscous flow.
Pumping in a molecular flow
For a pump to create suction, it needs enough molecules around for the domino-chain behavior of a viscous flow, so in molecular flow a pump cannot pull air. But if that pump Can't suck, what does it do? It traps. Like the goal on an air hockey table traps the puck when it flies in. But there's nobody knocking molecules towards the pump like there would be in air hockey, It's random.
How likely is a molecule to randomly fly into a pump that traps? That depends on how big the tube is leading to the pump.
Tube Size and Molecular Conductance
A long/narrow tube restricts gas flow more than a wide/short tube. The rate of gas flow through a tube is called the tube's conductance; the long/narrow tube has the lower conductance of the two.
Sadly adding a bigger pump to suck faster though the tiny tube only works in viscous flow. In molecular flow, the pump will only ever remove molecules at the rate they naturally fly through the smallest/longest tube in leading to the pump. The lowest conductance point sets the pace. =(
Mixing Gasses: Partial pressures
...