...
Ah but what happens when we add more molecules to the box? All of those little bounces add up. And that's pressure: the push from all the molecules. Add enough molecules and the pressure will rise so high that the box might pop!
Now what happens if we remove, instead of add, molecules? Vacuum.
Vacuum
When the pressure in the box is lower than the air around us, we call it a relative vacuum.
When there are absolutely no molecules whatsoever in a space, we call it a perfect vacuum.
but a perfect vacuum never happens. All vacuum chambers leak. And even in the furthest reaches of space, a stray molecule here and there flies past; though it probably won't hit another molecule for many lifetimes.
Now, getting into the nitty gritty:
Behaviors of gasses
Distance between bounces: Mean Free Path
The average distance that a gas molecule can travel before colliding with another gas molecule is called the mean free path.
as pressure drops, mean free path increases, because there are less molecules to run into. This effects the behavior of gasses, discussed below:
...
Gasses can act very differently depending on the the length of the mean free path. But let's start with what we're used to:
...
the air around us.
Behaviors of gasses
The air around us and domino chains
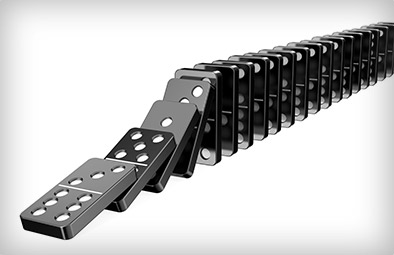
Remember: molecules are tiny, and there's a lot of them in the air around you—there are more molecules within a literal hair's width of a pinhead than one could possibly count. And what are they doing? Bouncing into each other. Knock enough molecules in one direction and those molecules will knock into the next like dominoes, and that's pretty much how sound happens
Hold your hand a few inches in front of your mouth away and say "pump". Do you feel that air hitting your hand? How many molecules was that? were all of the molecules that hit your hand from your mouth/lungs? The first molecules to hit your hand were already between your mouth and you hand when your
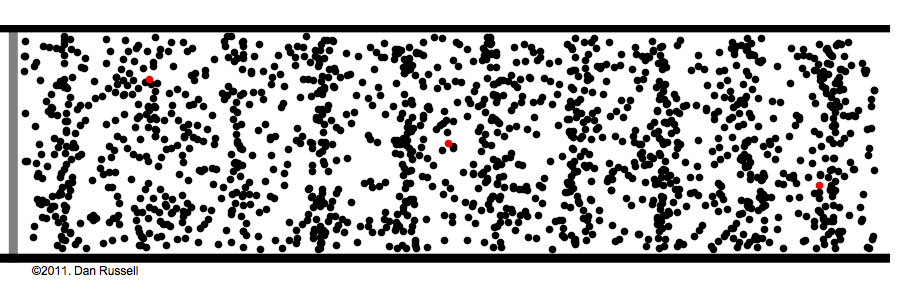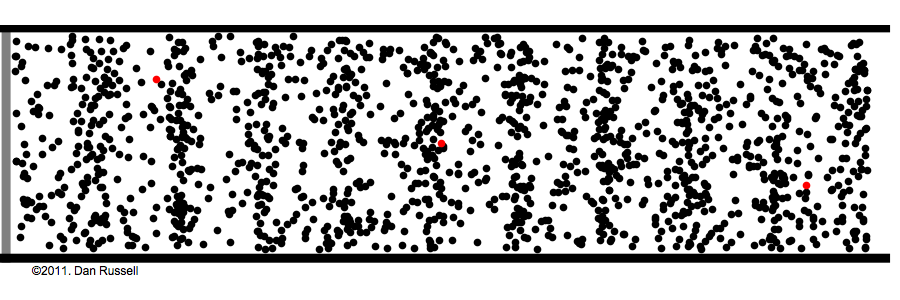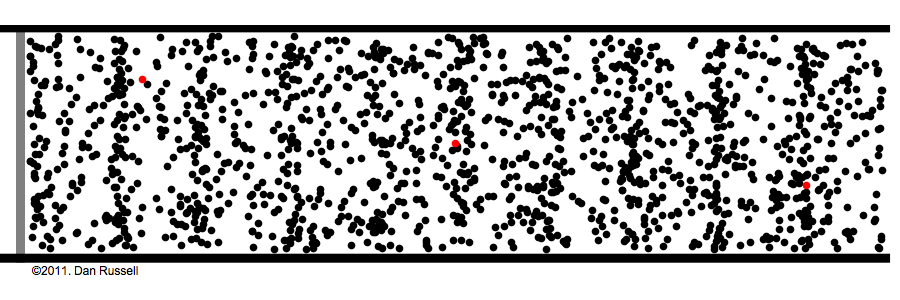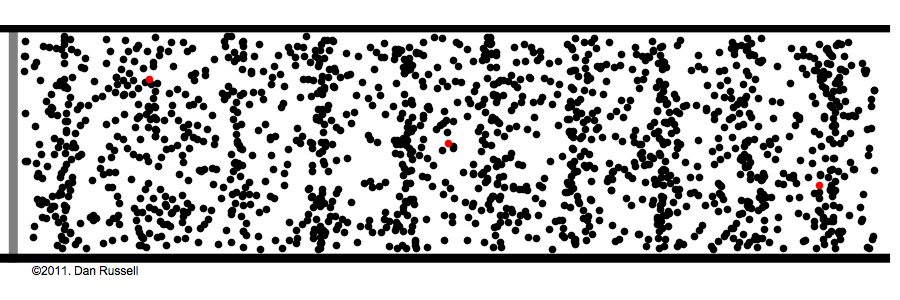
Under normal atmospheric conditions– gas molecules are constantly running into each other and bouncing off. This is how sound waves work:
Sound is waves of rapid pressure change, and no molecules need to travel from the speaker to the ear for us to hear. See Watch the red dots in the above animation. The speaker pushes some molecules into motion, that hit the next molecule in the way, that again hit the next, and so on and so forth—It's like dominoes.
In fact, when you blow out a candle it's not the molecules from your lungs that put out the flame, there are too many air molecules in the way; your lung-air molecules instead knock forward the air molecules in front of your mouth, and those molecules knock forward the air molecules above the cake, which in turn knock the air molecules in the flame away from the wick, taking their heat with them, extinguishing the flame.
Though if you keep blowing those lung air-molecules will
...
, see how they just oscillate back and forth? Now compare that to the smoke going back and forth with the sound waves, same thing:
The air moved quick enough to extinguish the flame, but there is no direction of flow, unlike when blowing out a flame:
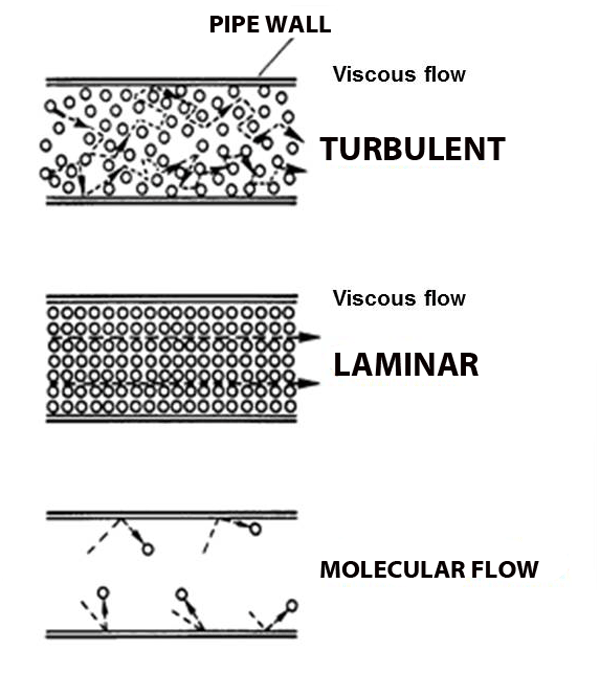
It is the continuous stream from behind that makes the air flow from one place to another—it's like pushing a crowd from the back. Keep pushing and it keeps flowing, as long as it has somewhere to go. We call this behavior viscous flow, and it's what we're used to.
Pumps create flow in just the opposite way with suction: remove some molecules and the ones next to it fall into the
...
empty space left behind, and just like the dominoes, that next molecule leaves it's own void behind
...
, which gets filled by other molecules, and so on and so forth.
...
Viscous flow depends on there being enough molecules around for domino chains to happen.what happens when the pressure gets so low that molecules hardly run into each other? Molecular flow.
Flowing through a pipe
Viscous flow: like a river
...
And pumps create suction in just the opposite way: remove some molecules and the ones next to it fall into the void, leaving their own void behind them, which gets filled by other molecules, and so on and so forth. This domino-chain like behavior is called viscous flow, and it's what we're used to.
But what happens when the pressure gets so low that molecules hardly run into each other? Molecular flow.
...
Molecular Flow: air hockey analogy
...