...
Now, getting into the nitty gritty:
Behaviors of gasses
Mean Free Path: bounce distance
The average distance that a gas molecule can travel before colliding with another gas molecule is called the mean free path.
...
as pressure drops, mean free path increases, because there are less molecules to run into. This effects the behavior of gasses, discussed below:
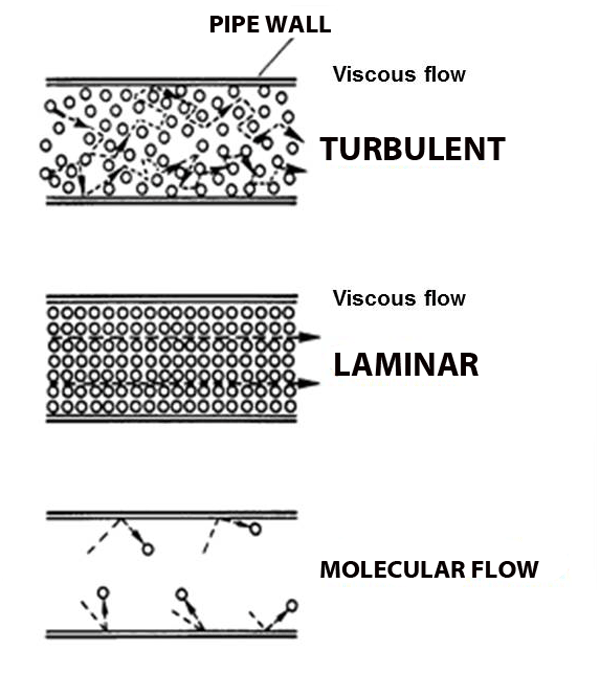
Viscous flow: like a river
Under normal atmospheric conditions– gas molecules are constantly running into each other (short mean free path) and bouncing off.
...
But what happens when the pressure gets so low that molecules hardly run into each other? Molecular flow.
Molecular Flow: air hockey analogy
Imagine the world's largest air hockey table, you place a few pucks on the table, and hit them in random directions. They'll hit each other sometimes, but they're much more likely to hit the wall. And it's just as likely to bounce back into your own goal as it is to bounce into the opponent's goal. Now replace the flat of the table with the space inside a vacuum chamber, and replace the pucks with molecules; this is molecular flow: when a molecule is more likely to hit a chamber wall than it is to hit another molecule, and unlike viscous flow, there is no general directionality to the flow (see figure above).
...