...
Now, getting into the nitty gritty:
Behaviors of gasses
Mean Free Path
The average distance that a gas molecule can travel before colliding with another gas molecule is called the mean free path.
as pressure drops, mean free path increases, because there are less molecules to run into. This effects the behavior of gasses, discussed below:
Viscous flow
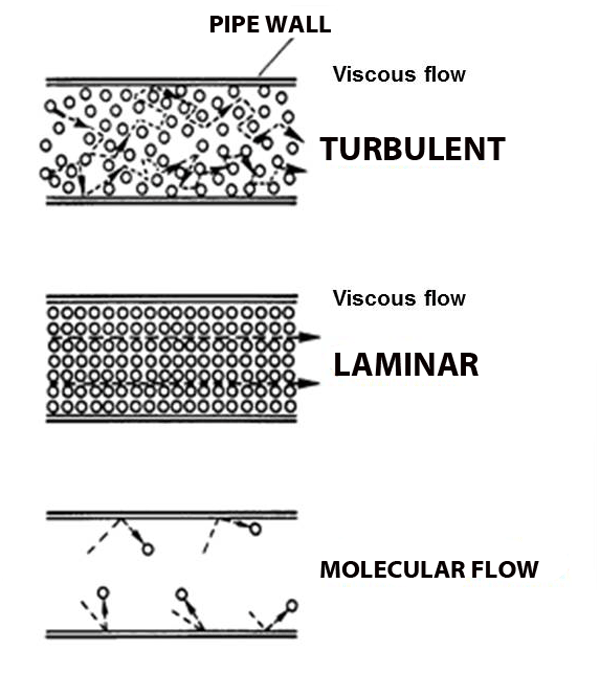
Under normal atmospheric conditions– gas molecules are constantly running into each other (short mean free path) and bouncing off.
In fact, when you blow out a candle it's not the molecules from your lungs that put out the flame, there are too many air molecules in the way; your lung-air molecules merely knock forward the air molecules in front of your mouth, which knock forward the air molecules above the cake, which in turn knock the air molecules in the flame away from the wick, taking their heat with them, and extinguishing the flame. And pumps create suction in just the opposite way: remove some molecules and the ones next to it fall into the void, leaving their own void behind them, which gets filled by other molecules, and so on and so forth. This domino-chain like behavior is called viscous flow, and it's what we're used to.
But what happens when the pressure gets so low that molecules hardly run into each other? Molecular flow.
Molecular Flow
Imagine the world's largest air hockey table, you place a few pucks on the table, and hit them in random directions. They'll hit each other sometimes, but they're much more likely to hit the wall. And it's just as likely to bounce back into your own goal as it is to bounce into the opponent's goal. Now replace the flat of the table with the space inside a vacuum chamber, and replace the pucks with molecules; this is molecular flow: when a molecule is more likely to hit a chamber wall than it is to hit another molecule, and unlike viscous flow, there is no general directionality to the flow (see figure above).
Pumping in a molecular flow
For a pump to create suction, it needs enough molecules around for the domino-chain behavior of a viscous flow, so in molecular flow a pump cannot pull air. But if that pump Can't suck, what does it do? It traps. Like the goal on an air hockey table traps the puck when it flies in. But there's nobody knocking molecules towards the pump like there would be in air hockey, It's random.
How likely is a molecule to randomly fly into a pump that traps? That depends on how big the tube is leading to the pump.
Tube Size and Molecular Conductance
A long/narrow tube restricts gas flow more than a wide/short tube. The rate of gas flow through a tube is called the tube's conductance; the long/narrow tube has the lower conductance of the two.
Sadly adding a bigger pump to suck faster though the tiny tube only works in viscous flow. In molecular flow, the pump will only ever remove molecules at the rate they naturally fly through the smallest/longest tube in leading to the pump. The lowest conductance point sets the pace. =(
Mixing Gasses: Partial pressures
So gasses mix. How does mixing gasses change pressure? The pressures add up. Here's an example:
...
In the mixed chamber the oxygen and nitrogen are still contributing the same pressures they had when they were separate. Those contributing pressures are called partial pressures. Oxygen's partial pressure in the mixed chamber is 2 units of pressure.
Gasses in Vacuum Chambers
Stuck on walls: Condensation and Evaporation
Ok, so you know how I said molecules bounce off walls? Not actually true. I lied to keep it simple, but I think you're ready for the truth: It turns out that any time a gas molecule hits a surface it actually sticks to that surface, just like how your breath fogs the mirror; this called adsorption (not to be confused with absorption).
And also just like fog on a mirror vanishes over time, those stuck molecules will eventually jump back off of the surface (I.e. evaporate, outgas, desorb), flying of in a random direction just as fast as it was when it hit the surface.
How long does the molecule stay stuck before desorbing from the surface?
Well that depends on the molecule, the temperature, and the what the surface is made of; and it can happen anytime between almost instantly and basically never.
If a type molecule tends to jump off quickly, from a given surface at a given temperature, it will find it's way into a pump pretty quickly, and the pressure will drop. And if a type molecule tends to jump off rarely, from a given surface at a given temperature, the pressure also goes down, it's not even contributing the the pressure, because it's not a gas, it's trapped on the surface!
The trouble for lowing pressure comes when a molecule jumps around enough to raise the pressure, but not enough to easily find it's way into the pump.
Contamination: molecules sticking to walls intermittently
main culprits
...
water - from moisture in the air
hydrocarbons (oils, plastics)
you know what's really oily? You.
how to defeat contamination
...
Prevent it!
use gloves, change them frequently
...
nitrogen gas has no water vapor, unlike air.
...
Bake it
making the chamber hot will get help those sticky molecules (water and oil) move along faster
detailed discussion the see pages on baking
Leaks
all vacuum chambers leak
really big leaks can be heard, usually from something like a mechanical connection that wasn't tightened
...
for detailed discussion see the Leak Checking pages
Time to talk numbers
Units
...
pressure: torr,
...
psi
gas flow rate: liters per second
Log Scales (making numbers lie)
not this log scale:
it's math. sorry.
linear vs logarithmic
UHV: how teeny tiny
...
Vacuum Chambers
it's just a box for holding nothing.
...




/GettyImages-1061851344-670639e17b314169bf6f62ba09bfd259.jpg)
