...
When a gas molecule bounces off of something it pushes on that something. If that something is another gas molecule that other molecule goes flying off. If that something is much bigger, for example a metal box, the molecule will push on the box just the same, but the box will hardly budge, it's way too big.
Ah but what happens when I we add more molecules to the box? All of those little bounces add up. And that's pressure: the push from all the molecules. Add enough molecules and the pressure will rise so high that the box might pop!
...
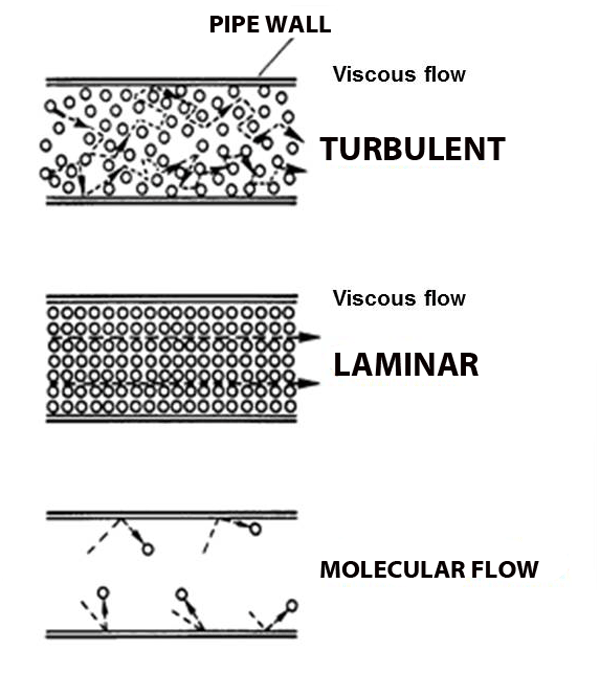
But what happens when the pressure gets so low that molecules hardly run into each other? Molecular flow.
Molecular Flow
Imagine the world's largest air hockey table, you place a few pucks on the table, and hit them in random directions. They'll hit each other sometimes, but they're much more likely to hit the wall. And it's just as likely to bounce back into my your own goal as it is to bounce into the opponent's goal. Now replace the flat of the table with the space inside a vacuum chamber, and replace the pucks with molecules; this is molecular flow: when a molecule is more likely to hit a chamber wall than it is to hit another molecule, and unlike viscous flow, there is no general directionality to the flow (see figure above).
...
In the mixed chamber the oxygen and nitrogen are still contributing the same pressures they had when they were separate. Those contributing pressures are called partial pressures. Oxygen's partial pressure in the mixed chamber is 2 units of pressure.
...
Stuck on walls: Condensation and Evaporation
ok, so you know how i I said molecules bounce off walls? Not actually true. I lied to keep it simple. Sorry... , but I think you're ready for the truth though: It turns turns out that any time a gas molecule hits a surface it actually sticks to that surface, just like how your breath fogs the mirror; this called adsorption (not to be confused with absorption).
but eventually that stuck molecule just jumps off (it desorbs, And also just like fog on a mirror vanishes over time, those stuck molecules will eventually jump back off of the surface (I.e. it evaporates, outgasses, desorbs), flying of in a random direction , flying just as fast as it was when it hit that the surface. This is the outgassing referred to earlier.
How long until it desorbs does the molecule stay stuck before desorbing from the surface? Well molecules can fly off anytime between almost instantly, to basically never, and that depends on the molecule, the temperature, and the what the material surface is made of.
Rate of evaporation: Vapor pressure
outgassing
contamination
; and it can happen anytime between almost instantly and basically never.
If a type molecule tends to jump off quickly, from a given surface at a given temperature, it will find it's way into a pump pretty quickly, and the pressure will drop. And if a type molecule tends to jump off rarely, from a given surface at a given temperature, the pressure also goes down, it's not even contributing the the pressure, because it's not a gas, it's trapped on the surface!
The trouble for pressure comes when a molecule jumps around enough to raise the pressure, but not enough to easily find it's way into a pump.
contamination: molecules sticking to walls intermittently
main culprits:
water - from moisture in the air
...

/GettyImages-1061851344-670639e17b314169bf6f62ba09bfd259.jpg)