| Table of Contents |
|---|
This is a beginners beginner guide to vacuum theory for beginners, and it assumes the reader knows nothing about vacuums, and avoids the use of math and units. It introduces aims to incrementally introduce concepts and defines define some of the language used to discuss of vacuum technology, and is intended to lay laying the groundwork for deeper understandinglearning. This Guide guide starts off way zoomed out and oversimplified, building off itself as it goes, introducing new terms along the way, ending and ends still zoomed out and oversimplified --but –but a bit less so.
There's so much more depth behind these subjects than document acknowledges. concepts than acknowledge here! More technical discussions discussion and descriptions can be found within the other pages of LCLS Vacuum Support Training. But , within textbooks, and most of all within your coworkers (go talk with them!).
Side note: many of the pictures are linked to external material , including videos, lessonsarticles, slideshows, and product pages; click . Click to explore, but though they aren't necessary, and may be tangential or go way beyond the scope of this introductory guide.
Happy reading!
Starting at the bottom, Setting the scene
Atoms
Atoms, totally a thing! they're small. really small. a million of them fit across a single human hair. tiny, but they still take up space.
...
they often like to group up with other atoms to form molecules.
Gasses
Hey so gasses exist. You're breathing some right now.
what are gasses made of? molecules. Usually as a mix of different molecule types. Air is a mix of over ten types (mostly nitrogen and oxygen). People work very hard to collect gasses that aren't mixed.
what are those gas molecules doing? mostly just bouncing around. –off each other, off the walls
...
BUT: the number of molecules in a room depends on the pressure of the gas in the room: fewer molecules = lower pressure.
Gas Pressure
When a gas molecule bounces off of something it pushes on that something. If that something is another gas molecule that other molecule goes flying off. If that something is much bigger, for example a metal box, the molecule will push on the box just the same, but the box will hardly budge, it's way too big.
...
Now what happens if we remove, instead of add, molecules? Vacuum.
Vacuum
When the pressure in the box is lower than the air around us, we call it a relative vacuum.
...
Now, getting into the nitty gritty:
Zooming in,
...
Details and
...
Definitions
Behaviors of gasses
mean free path
The average distance that a gas molecule can travel before colliding with another gas molecule is called the mean free path.
...
as pressure drops, mean free path increases, because there are less molecules to run into. this effects the behavior of gasses.
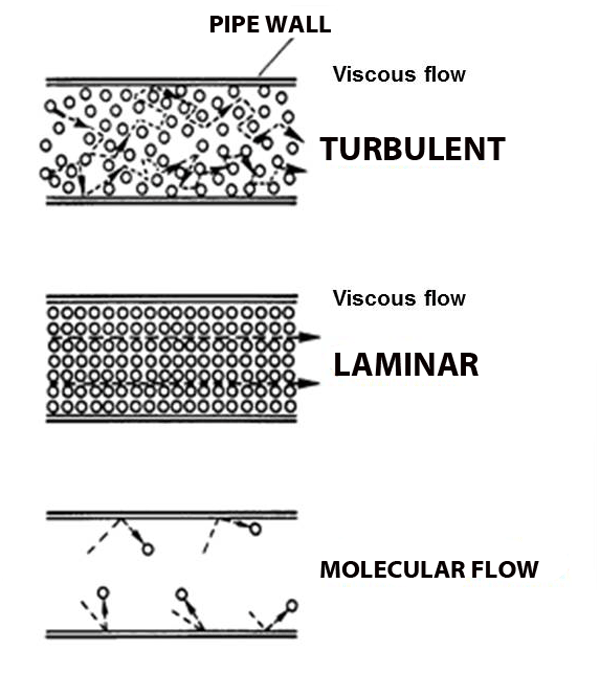
Viscous flow
Under normal atmospheric conditions-- conditions– gas molecules are constantly running into each other (short mean free path) and bouncing off.
...
But what happens when the pressure gets so low that molecules hardly run into each other? Molecular flow.
Molecular Flow
When a molecule is more likely to hit a chamber wall than it is to hit another molecule the gas starts behaving in strange ways: wind does not blow and pumps cannot suck. We call this behavior molecular flow (see figure above).
Imagine the world's largest air hockey table, with a few pucks zipping about. Even if I manage to knock one puck, it is unlikely to hit any other pucks on it's way to the goal. That is molecular flow. Wind does not blow and pumps cannot suck because there are not enough molecules around for domino-chain effects to occur (like blowing out a candle).
Vacuum Chambers
The Chamber itself
just a box for holding nothing.
...
perhaps with holes to attach stuff, like pumps.
Pumping
A pump can't pull air after gasses enter molecular flow. But if that pump Can't suck, what does it do? It traps. Like the goal on an air hockey table traps the puck when it flies in.
So how likely is a molecule to fly into a pump that traps? That depends on how big the tube is leading to the pump.
Conductance
A long/narrow tube restricts gas flow more than a wide/short tube. The rate of gas flow through a tube is called the tube's conductance; the long/narrow tube has the lower conductance of the two.
Sadly adding a bigger pump to suck faster though the tiny tube only works in viscous flow. In molecular flow, the pump will only ever remove molecules at the rate they naturally fly through the smallest/longest tube in leading to the pump. The lowest conductance point sets the pace. =(
different pumps for different pressures:
Pumps come in many different shapes, sizes, and types.
...
for more details on how they actually work, see the confluence page on vacuum pumps. and for that matter, also see the page on gauges, we layer those too.
Pressure,
...
Revisited:
...
This time it's chambers.
Partial pressure
So gasses mix. How does mixing gasses change pressure? The pressures add up. For example:
...